In a chemistry class, the teacher placed 0.5 g of porous steel wool
In a chemistry class, the teacher placed 0.5 g of porous steel wool, composed mostly of iron (Fe), inside a small heat-resistant quartz tube. She then used silicone hoses to connect the quartz tube to 2 airtight glass syringes (see figure). Each syringe contained 8 mL of air, and the total volume of air in the closed apparatus was 20 mL.
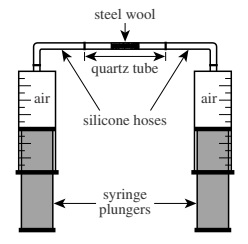
A Bunsen burner was then used to heat the contents of the quartz tube for 2 min. During heating, the plungers were moved up and down to pass the air back and forth through the steel wool. The total volume of gas in the apparatus steadily declined over the 2 min. Once the apparatus and its contents returned to room temperature, the total volume of gas in the apparatus was 16 mL.
The teacher asked each of 4 students to explain what occurred during the demonstration.
Student 1
During heating, the Fe in the steel wool reacted with all the N2 in the air to form solid iron nitride (FeN), which was deposited on the steel wool. Air contains about 20% N2 by volume. As a result of the reaction, the total volume of gas in the apparatus decreased by about 20%, so almost all the gas remaining in the apparatus was O2.
Student 2
During heating, the Fe in the steel wool reacted with some of the O2 in the air to form solid iron oxide (Fe2O3), which was deposited on the steel wool. Air contains about 80% O2 by volume. As a result of the reaction, the total volume of gas in the apparatus decreased by about 20%. Almost all the gas remaining in the apparatus was a mixture of about 75% O2 and 25% N2 by volume.
Student 3
Student 2 is correct, except that (1) the Fe in the steel wool reacted with all the O2 in the air and (2) air contains about 20% O2 by volume. After the reaction, almost all the gas remaining in the apparatus was N2.
Student 4
During heating, the Fe in the steel wool reacted with all the CO2 in the air to form solid iron carbonate (FeCO3), which was deposited on the steel wool. Air contains about 20% CO2 by volume. As a result of the reaction, the total volume of gas in the apparatus decreased by about 20%, so almost all the gas remaining in the apparatus was O2.
28. Air contains less than 1% argon by volume. This information weakens the explanations given by which of the students, if any?
F. Students 1 and 2 only
G. Students 3 and 4 only
H. All of the students
J. None of the students
29. Silicone hoses were most likely used to connect the quartz tube to the syringes because silicone has which of the properties listed below?
I. Strong resistance to heat
II. Low chemical reactivity
III. High solubility in water
A. I and II only
B. I and III only
C. II and III only
D. I, II, and III
30. Based on Student 4’s explanation, during the demonstration, did the percent CO2 by volume in the apparatus increase or decrease, and did the percent O2 by volume in the apparatus increase or decrease?
percent CO2 by volume | percent O2 by volume
F. increase increase
G. increase decrease
H. decrease decrease
J. decrease increase
31. Which of the students would be likely to agree that, by volume, air contains more O2 than N2 ?
A. Students 1 and 2 only
B. Students 1 and 3 only
C. Students 1, 2, and 4 only
D. Students 1, 3, and 4 only
32. Based on Student 3’s explanation, the reaction that occurred during the demonstration would be represented by which of the following balanced chemical equations?
F. 2Fe2O3 → 4Fe + 3O2
G. 2FeN → 2Fe + N2
H. 4Fe + 3O2 → 2Fe2O3
J. 2Fe + N2 → 2FeN
33. Which of the students, if any, would be likely to agree that at the end of the demonstration, the gas remaining in the apparatus was at least 20% N2 by volume?
A. Student 2 only
B. Students 2 and 3 only
C. All of the students
D. None of the students
34. In a chemical reaction, the limiting reactant is the reactant that is in the shortest supply and thus limits the amount of product that can be produced. Which student would be the most likely to agree that the limiting reactant during the demonstration was the iron in the steel wool?
F. Student 1
G. Student 2
H. Student 3
J. Student 4
Answers
28. The best answer is J. According to the passage, none of the students mentioned argon. All four students referred to “almost all the gas remaining . . .,” which suggests that other gases, such as argon, could be present. The fact that air contains less than 1% argon by volume does not weaken the explanation given by any of the students.
29. The best answer is A. Because the quartz tube was heated with a Bunsen burner, it was important that the silicone hoses had a strong resistance to heat. Because a chemical reaction was taking place when the steel wool was heated, it was important that the silicone hoses have a low chemical reactivity. No water was used in the experiment, nor was water generated, and therefore the solubility of the silicone hoses in water would not be important.
30. The best answer is J. According to Student 4, the Fe reacted with CO2 in the air, and after the reaction, almost all the remaining gas was O2. Because the CO2 was used up, the percent CO2 by volume decreased. Because the remaining gas was nearly all O2, the percent O2 by volume increased as the CO2 reacted.
31. The best answer is C. According to Student 1, air contains 20% N2 by volume, and after the N2 reacted with the Fe, almost all the gas remaining was O2. It follows that Student 1 believes air is approximately 80% O2. Student 2 states that air contains about 80% O2. Student 3 states that air contains 20% O2 by volume, and after the O2 reacted with the Fe, almost all the gas remaining was N2. It follows that Student 3 believes air is approximately 80% N2. According to Student 4, air contains about 20% CO2 by volume, and after the CO2 reacted with the Fe, almost all the gas remaining was O2. It follows that Student 4 believes air is approximately 80% O2. Students 1, 2, and 4 all believe that air contains approximately 80% O2 by volume and therefore would agree that, by volume, air contains more O2 than N2.
32. The best answer is H. In order to answer this item, the examinee needs to understand how reactants and products are placed in chemical equations. According to Student 3, the Fe reacted with the O2 to form Fe2O3. Fe and O2 are reactants and
will appear on the left side of the equation. Fe2O3 is the product and will appear on the right side of the equation. F is incorrect; the reactants and product are on the wrong sides of this equation.
33. The best answer is B. According to the passage, Students 1 and 4 claim that almost all the gas remaining is O2. Student 2 claims that almost all the remaining gas is a mixture of about 75% O2 and 25% N2. Student 3 claims that almost all the remaining gas is N2. Students 2 and 3 would agree that the gas remaining was at least 20% N2 by volume.
34. The best answer is G. According to Student 1, the Fe reacted with all the N2, indicating that N2 is the limiting reagent, and Fe is present in excess. Student 2 stated that the Fe reacted with some of the O2, indicating the Fe is the limiting reagent and O2 is present in excess. Student 3 stated that the Fe reacted with all the O2, indicating that O2 is the limiting reagent and Fe is present in excess. Student 4 stated that the steel wool reacted with all the CO2, indicating that CO2 was the limiting reagent and Fe was present in excess. Only Student 2 would likely agree that Fe was the limiting reagent.