For gas atoms in a state of random motion, the mean free path
For gas atoms in a state of random motion, the mean free path, λ, is the average distance a gas atom will travel between collisions with other gas atoms. This distance depends upon the diameter of the gas atom, d, the volume of the gas, V, and the number of atoms of the gas, N.
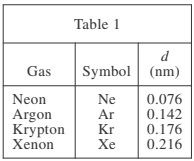
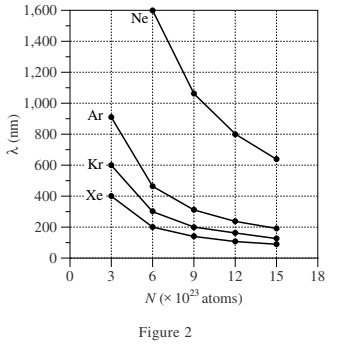
Table 1 lists the name, symbol, and value of d (in nanometers, nm) for each of 4 gases. Figure 1 shows, for each gas, at 293 kelvins (K), how λ (in nm) varies with V (in liters, L) in a sample with N = 6 × 1023 atoms of the gas. Figure 2 shows, for each gas, at 293 K, how λ varies with N in a sample with V = 25 L.
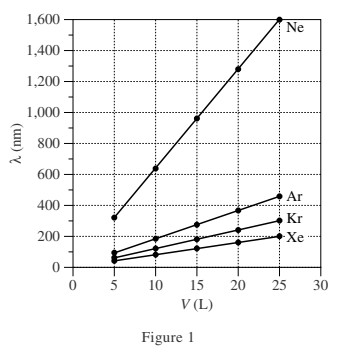
1. According to Figure 2, what is the order of gas samples from shortest λ to longest λ for N = 15 × 1023 atoms?
- Ne, Ar, Kr, Xe
- Ne, Kr, Ar, Xe
- Xe, Ar, Kr, Ne
- Xe, Kr, Ar, Ne
2. According to Figure 2, doubling the Ne sample size from 6 × 1023 atoms to 12 × 1023 atoms effectively multiplies λ for Ne by a factor of:
- 1/4
- 1/2
- 2
- 4
3. Consider 2 Kr samples at 293 K, each with N = 6 × 1023 atoms, but one with V = 25 L and the other with V = 50 L. Based on Figure 1, λ for the 50 L sample would most likely be how many times as great as λ for the 25 L sample?
- 1/4
- 1/2
- 2
- 4
4. Based on Figure 1, for the Xe and Ar gas samples with V = 20 L, compared to λ for Xe, approximately how much longer is λ for Ar ?
- 50 nm
- 100 nm
- 150 nm
- 200 nm
5. The collision frequency is defined as the number of collisions between gas atoms per second. Consider the 5 L and 25 L Xe samples represented in Figure 1. Assuming the Xe atoms have the same average speed in both samples, in which sample would the collision frequency more likely be higher?
- In the 5 L sample; Xe atoms in the 5 L sample travel, on average, shorter distances between collisions and therefore collide more often.
- In the 5 L sample; Xe atoms in the 5 L sample travel, on average, longer distances between collisions and therefore collide more often.
- In the 25 L sample; Xe atoms in the 25 L sample travel, on average, shorter distances between collisions and therefore collide more often.
- In the 25 L sample; Xe atoms in the 25 L sample travel, on average, longer distances between collisions and therefore collide more often.
6. For a particular sample of radon (Rn) gas in a 25 L container at 293 K, λ is approximately 320 nm. If d for Rn is 0.240 nm, then, based on Table 1 and Figure 2, approximately how many Rn atoms are most likely in this sample?
- Less than 6 × 1023
- Between 6 × 1023 and 9 × 1023
- Between 9 × 1023 and 12 × 1023
- More than 12 × 1023
Answers
1. The correct answer is D.
According to Figure 2, when N = 15 × 1023 atoms, Xe had the shortest λ, followed by Kr, Ar, and Ne.
2. The correct answer is B.
According to Figure 2, when N for Ne was equal to 6 × 1023 atoms, λ = 1,600 nm and when N for Ne was equal to 12 × 1023 atoms, λ = 800 nm. When the Ne sample size doubled, λ was reduced by half.
3. The correct answer is C.
According to Figure 1, as V increases, λ also increases. When V = 5 L, λ was approximately 50 nm and when V = 10 L, λ was approximately 100 nm. Based on this trend, if V is doubled from 25 L to 50 L, then λ should also double.
4. The correct answer is D.
According to Figure 1, when V = 20 L, λ for Xe is approximately 175 nm and λ for Ar is approximately 375 nm. Thus, λ for Ar is approximately 200 nm longer than λ for Xe.
5. The correct answer is A.
If the atoms have the same average speed, then the sample with the shortest λ (distance between collisions) will have the highest collision frequency. Figure 1 shows that Xe atoms in the 5 L sample have a shorter λ than atoms in the 25 L sample.
6. The correct answer is A.
According to Figure 2, for any given λ, the lower the value of d, the greater the value of N. When λ = 320 nm for Xe, there are fewer than 6 × 1023 atoms of Xe present. Because d for Rn is greater than that for Xe, it follows that there are also fewer than 6 × 1023 atoms of Rn present.