A high concentration of dissolved nickel (Ni2+) in wastewater is an environmental concern
A high concentration of dissolved nickel (Ni2+) in wastewater is an environmental concern. Students studied the removal of Ni2+ from wastewater, using an aqueous Ni2+ solution as a model of wastewater.
In water, hydroxide (OH-) reacts with Ni2+ to form nickel hydroxide monohydrate [Ni(OH)2.H2O]. The balanced chemical equation for this reaction is
Ni2+ + 2OH- + H2O → Ni(OH)2.H2O
Because the monohydrate is a solid, it can be filtered from the solution. Some of the solid will eventually dissolve if it is left in contact with the solution.
The students did 2 experiments to study how reaction time and filtration method affected the removal of Ni2+ from the aqueous Ni2+ solution.
Experiment 1
In each of Trials 1-3, Steps 1-4 were performed:
1. Thirty-two mL of aqueous 1.0 mole/L OH- solution and 260 mL of aqueous 0.060 mole/L Ni2+ solution were poured into the same flask.
2. The mixture was stirred at 22°C for 10 min, 3 days, or 7 days.
3. Solid monohydrate was recovered by standard filtration (see Figure 1).

4. The concentration of Ni2+ in the filtrate, CNF, was determined, in milligrams of Ni2+ per kilogram of solution (mg/kg).
Experiment 2
In each of Trials 4-6, Steps 1-4 in Experiment 1 were performed except that in Step 3, solid monohydrate was recovered by vacuum filtration (see Figure 2).
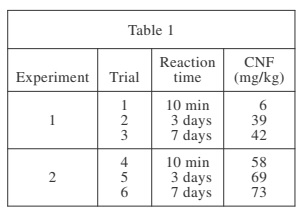
The results of Experiments 1 and 2 are shown in Table 1.
1. If a reaction time of 2 days had been tested in Experiment 1, the CNF would most likely have been:
- less than 6 mg/kg.
- between 6 mg/kg and 39 mg/kg.
- between 39 mg/kg and 42 mg/kg.
- greater than 42 mg/kg.
2. Based on the results of Experiments 1 and 2, what combination of reaction time and filtration method resulted in the lowest concentration of dissolved nickel in the filtrate?
reaction time - filtration method
- 10 min - standard
- 7 days - standard
- 10 min - vacuum
- 7 days - vacuum
3. Was the net force exerted on the mixture in the funnel more likely greater in Trial 3 or in Trial 6 ?
- Trial 3, because the filtration apparatus was connected to a vacuum pump.
- Trial 3, because the filtration apparatus was not connected to a vacuum pump.
- Trial 6, because the filtration apparatus was connected to a vacuum pump.
- Trial 6, because the filtration apparatus was not connected to a vacuum pump.
4. In each trial, the students performed which of the following chronological sequences of steps?
- Measuring the CNF; recovering the solid by filtration; mixing the Ni2+ and the OH- solutions
- Mixing the Ni2+ and the OH- solutions; recovering the solid by filtration; measuring the CNF
- Recovering the solid by filtration; measuring the CNF; mixing the Ni2+ and the OH- solutions
- Recovering the solid by filtration; mixing the Ni2+ and the OH- solutions; measuring the CNF
5. A student predicted that when solid monohydrate is recovered by vacuum filtration, a greater CNF will result for a reaction time of 3 days than for a reaction time of 10 min. Do the data in Table 1 support this prediction?
- No; Trial 1 had a greater CNF than did Trial 2.
- No; Trial 5 had a greater CNF than did Trial 4.
- Yes; Trial 1 had a greater CNF than did Trial 2.
- Yes; Trial 5 had a greater CNF than did Trial 4.
6. In how many of the 6 trials was nickel hydroxide monohydrate recovered by standard filtration after OH- and Ni2+ had been allowed to react for at least 3 days?
- 1
- 2
- 4
- 6
7. Based on the balanced chemical equation in the passage, as 6 OH- ions are consumed, how many formula units of Ni(OH)2.H2O are produced?
- 3
- 6
- 12
- 18
Answers
1. The correct answer is B.
According to Table 1, in Experiment 1, as the reaction time increased, the CNF also increased. When the reaction time was 10 min, the CNF was 6 mg/kg, and when the reaction time was 3 days, the CNF was 39 mg/kg. If a reaction time of 2 days had been tested, then the CNF would most likely have been between 6 mg/kg and 39 mg/kg.
2. The correct answer is A.
According to Table 1, the lowest concentration of dissolved nickel in the filtrate was found in Trial 1 (CNF = 6 mg/kg). In Trial 1, the reaction time was 10 min and the standard filtration method was used.
3. The correct answer is C.
In order to answer this item, you must know that applying a vacuum will increase the net force on the mixture in the funnel. The net force exerted on the mixture was greater in Trial 6 than in Trial 3, because the vacuum filtration technique was used in Trial 6.
4. The correct answer is B.
According to the passage, the solutions were mixed, the mixture was stirred, the solid was recovered by filtration, and then the CNF was measured.
5. The correct answer is D.
According to Table 1, when the reaction time was 3 days and vacuum filtration was used, the CNF was 69 mg/kg (Trial 5). When the reaction time was 10 min and vacuum filtration was used, the CNF was 58 mg/kg (Trial 4). The data do support the student’s prediction that the CNF will be greater for 3 days and vacuum filtration than for 10 min and vacuum filtration.
6. The correct answer is B.
According to the passage, only Trials 1, 2, and 3 used standard filtration. In Trial 2, the reaction time was 3 days, and in Trial 3, the reaction time was 7 days. The reaction time for Trial 1 was only 10 min.
7. The correct answer is A.
In order to answer this item, you must recognize that the stoichiometry of the balanced chemical equation shows that 2 hydroxide ions are needed to produce 1 formula unit of the monohydrate. If 6 hydroxide ions are used, then 3 formula units of the monohydrate will be produced.